Preventing Adverse Drug Reactions with Pharmacogenetic Testing
 Dec, 24 2025
Dec, 24 2025
Every year, hundreds of thousands of people in the UK and across the world are hospitalized because of unexpected side effects from medications they were prescribed. These aren’t allergies or overdoses - they’re adverse drug reactions (ADRs), often caused by how a person’s genes process drugs. For many, it’s not bad luck. It’s biology. And now, there’s a way to see it coming before the first pill is even taken.
What Pharmacogenetic Testing Actually Does
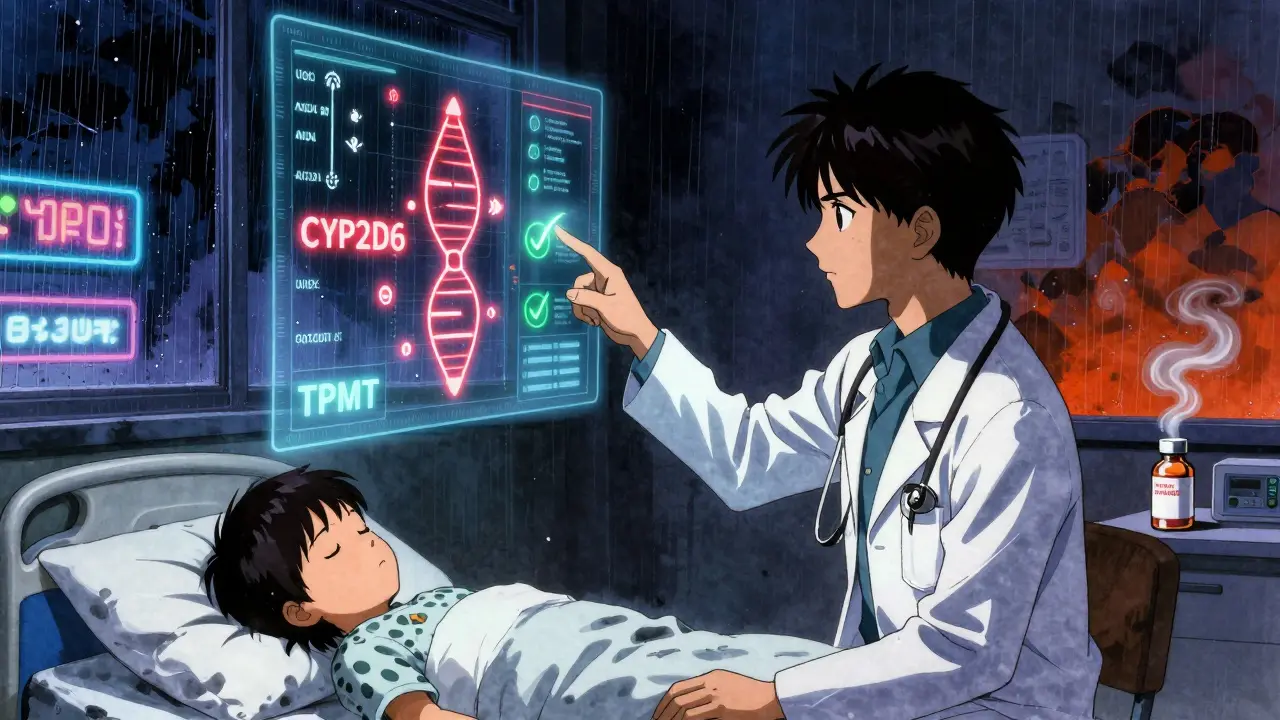
Pharmacogenetic testing looks at your DNA to find out how your body breaks down medicines. It’s not about predicting disease. It’s about predicting how you’ll respond to a drug. Some people metabolize drugs too fast - the medicine leaves their system before it can work. Others metabolize too slow - the drug builds up and causes toxicity. Your genes decide which group you’re in. Take clopidogrel, a common blood thinner after a heart attack. About 30% of people have a variant in the CYP2C19 gene that makes this drug useless for them. Without testing, they’re left with a false sense of security, risking a second heart attack. With testing, doctors switch them to a different drug - like prasugrel or ticagrelor - and avoid the danger entirely. Or consider codeine. It’s a painkiller that turns into morphine in the body. But some people have extra copies of the CYP2D6 gene. Their bodies convert codeine into morphine too quickly. In rare cases, this has led to fatal breathing problems in children after tonsil surgery. Testing can spot these ultra-rapid metabolizers before the prescription is written.The Landmark Study That Changed Everything
In 2023, a massive study called PREPARE - led by researchers at the University of Liverpool and involving nearly 7,000 patients across seven European countries - proved this isn’t just theory. It’s practice. Patients were tested for 12 key genes before being prescribed any of over 100 common medications. These included genes like CYP2D6, CYP2C19, TPMT, SLCO1B1, and HLA-B. The results? A 30% drop in serious adverse drug reactions. That’s not a small improvement. That’s a game-changer. What made this study different? It wasn’t done after someone got sick. It was done before any drugs were given. That’s called preemptive testing. And it works better than waiting for a reaction to happen - reactive testing only cuts ADRs by 15-20%.Which Genes Matter Most
Not every gene matters for every drug. But for common prescriptions, a handful make all the difference:- CYP2C19: Affects clopidogrel, antidepressants like citalopram, and proton pump inhibitors like omeprazole.
- CYP2D6: Impacts codeine, tramadol, tamoxifen, and many antidepressants.
- TPMT: Critical for azathioprine and mercaptopurine - used in autoimmune diseases and cancer. Without testing, patients can develop life-threatening bone marrow suppression.
- SLCO1B1: Predicts muscle pain from statins like simvastatin. A simple test can help avoid a painful, dangerous side effect.
- HLA-B*1502: A genetic marker that, if present, makes carbamazepine (used for epilepsy and bipolar disorder) extremely risky for people of Asian descent. Testing cuts the risk of Stevens-Johnson syndrome by 95%.
How It Works in Real Clinics
Getting tested isn’t complicated. A simple cheek swab or blood sample is sent to a lab. Results come back in 24 to 72 hours. In hospitals like those in the UK’s NHS, results are now integrated into electronic health records. When a doctor prescribes a drug like clopidogrel or simvastatin, a pop-up alert appears: "Patient has CYP2C19 poor metabolizer variant. Consider alternative therapy." This isn’t science fiction. It’s happening now. The University of Florida’s personalized medicine program has been doing this since 2012. They saw a 75% drop in ADR-related emergency visits among tested patients. The cost? Around $1.2 million to set up. The payback? Less than two years, thanks to fewer hospitalizations and fewer wasted prescriptions. In the UK, the NHS estimates ADRs cost £500 million a year in avoidable hospital stays. Pharmacogenetic testing could cut that significantly. And with the European Commission committing €150 million to roll this out by 2027, it’s only going to become more common.Why It’s Not Everywhere Yet
Despite the evidence, adoption is still slow - especially in primary care. Only 18% of GP practices in the UK have integrated pharmacogenetic testing. Why? First, many doctors don’t feel confident interpreting the results. A 2022 survey found only 37% of physicians felt comfortable using genetic data to adjust prescriptions. Training helps. A few hours of CME-accredited education can change that. Second, there’s confusion over what to do with "intermediate metabolizers" - people who fall between fast and slow. Guidelines from the Clinical Pharmacogenetics Implementation Consortium (CPIC) help, but they’re not always easy to apply in a 10-minute appointment. Third, cost. A full panel test runs between £150 and £300 in the UK. That’s not cheap. But compared to the £10,000 average cost of an ADR-related hospital admission, it’s a bargain. And the price is dropping. New point-of-care tests are being developed that could bring the cost under £50 by 2026.
Who Benefits the Most
Some groups see the biggest gains:- People on multiple medications: Polypharmacy increases ADR risk. Testing helps untangle which drug might be causing trouble.
- Patients with psychiatric conditions: Antidepressants and antipsychotics have high rates of side effects. One study showed a 40% reduction in side effects after genotype-guided prescribing.
- Cancer patients: Chemotherapy drugs like 5-FU and irinotecan can be deadly if metabolized poorly. Testing prevents severe toxicity in up to 10% of patients.
- Older adults: As liver and kidney function decline with age, genetic factors become even more important in drug dosing.
What’s Next
The future isn’t just about single genes. Researchers are now building polygenic risk scores - combining dozens of genetic markers to predict drug response with even greater accuracy. Early results show a 40-60% improvement over single-gene testing. The FDA has added 42 new gene-drug pairs to its list since 2022, bringing the total to 329. The European Medicines Agency now includes pharmacogenetic warnings in nearly a third of new drug labels. And the public? Most people are ready. In surveys, 85% say they’d be willing to get tested if their doctor recommended it. The big worry? Privacy. About one in three people fear their genetic data could be misused. That’s why secure, anonymized systems and strict data governance are just as important as the science.Is It Right for You?
You don’t need to get tested for every drug. But if you’re:- On long-term medication (especially for depression, epilepsy, heart disease, or cancer)
- Experiencing unexplained side effects
- Have family members who had bad reactions to the same drug
- Or are about to start a new high-risk medication
Is pharmacogenetic testing covered by the NHS?
Currently, the NHS doesn’t offer universal pharmacogenetic testing. But it does cover specific tests for high-risk cases - like TPMT testing before azathioprine or CYP2C19 testing before clopidogrel. Wider rollout is planned, especially as evidence grows and costs fall. Some hospital trusts already offer it for oncology and psychiatry patients.
How long does it take to get results?
In most hospital labs, results come back in 24 to 72 hours. Some private clinics offer faster turnaround - under 24 hours - but at a higher cost. As point-of-care testing improves, we’re likely to see results in under an hour by 2026.
Can I get tested without a doctor’s order?
Yes - direct-to-consumer tests are available online. But be careful. Most of these only test a few genes and don’t provide clinical interpretation. Without a doctor’s guidance, you might misread your results and make unsafe changes to your medication. For safety and accuracy, always get tested through a healthcare provider.
Does this test reveal other health risks?
No. Pharmacogenetic tests look only at genes that affect how your body processes drugs. They don’t screen for diseases like cancer, Alzheimer’s, or heart disease. Your privacy is protected - the test is focused solely on medication safety.
What if I’ve already had a bad reaction to a drug?
It’s not too late. Testing after an adverse reaction can help explain why it happened and prevent it from happening again. Many doctors now recommend it for patients who’ve had unexplained side effects. It can also help identify safe alternatives.
Will my insurance cover this?
In the UK, private health insurance may cover pharmacogenetic testing if it’s deemed medically necessary. In the US, Medicare and Medicaid cover specific tests like TPMT and CYP2C19. Always check with your provider. Even if not covered, the cost is often less than a single emergency visit caused by a preventable reaction.

sagar patel
December 24, 2025 AT 19:49Pharmacogenetics is just the beginning of corporate medicine selling you genetic fear to upsell tests
Jason Jasper
December 26, 2025 AT 02:20I’ve had two bad reactions to antidepressants. My doctor didn’t even know this testing existed. It’s crazy we’re still flying blind.
Lindsay Hensel
December 26, 2025 AT 12:49This is precisely why personalized medicine must be treated as a human right-not a luxury. The science is undeniable, and the ethical imperative is clear.
Michael Dillon
December 28, 2025 AT 02:10So let me get this straight-you’re telling me we’ve known for decades that genes affect drug metabolism, but we’re only now acting on it because a fancy study proved it? Classic.
Gary Hartung
December 29, 2025 AT 22:53Let’s be honest-this isn’t science. It’s a marketing campaign disguised as progress. Who profits? Labs. Pharma. Insurance companies. Not patients. The real story? The FDA’s list of gene-drug pairs has grown because they’re legally required to warn-but not because it’s clinically urgent.
And don’t get me started on the ‘93.5% of people have a variant’ claim-that’s like saying ‘93.5% of people have lungs,’ and then charging them for an oxygen subscription.
Also, why is no one talking about the fact that these tests are mostly validated in European populations? What about African, Indigenous, or South Asian variants? The data is skewed. We’re building a precision medicine system on biased data.
And then there’s the privacy issue-your DNA isn’t just about drugs. It’s your identity. Once it’s in a database, it’s out there. Forever. And no, ‘anonymized’ doesn’t mean untraceable.
Yes, this saves money. But who’s paying the cost? The patient’s autonomy. The patient’s right to not know. The right to be treated as a person, not a data point.
I’m not against testing. I’m against the cult of genetic determinism wrapped in a white coat.
Ben Harris
December 31, 2025 AT 13:52My grandma took warfarin for 15 years without a problem but they told her she was a slow metabolizer so they switched her to apixaban and now she’s falling all the time
Maybe the test is wrong maybe the doctor misread it maybe she just got old
Why do we trust machines more than lived experience
Rick Kimberly
January 1, 2026 AT 11:49Thank you for this comprehensive and evidence-based overview. The PREPARE study’s 30% reduction in adverse reactions is compelling, particularly when contextualized against the £500 million annual NHS burden. The integration of results into EHRs-such as the pop-up alerts in UK hospitals-is a model worth scaling globally.
It’s also worth noting that the drop in emergency visits at the University of Florida was not merely a cost-saving measure; it was a reduction in human suffering. Patients avoided hospitalization, pain, and potential disability.
While cost and physician education remain barriers, the trajectory is clear: as point-of-care testing becomes more affordable and AI-assisted decision tools emerge, the standard of care will shift from reactive to preemptive. This is not just innovation-it’s evolution.
For those concerned about privacy, robust governance frameworks like GDPR and HIPAA, coupled with patient-controlled data portals, can mitigate risk. The question isn’t whether we should adopt this technology-it’s how quickly we can implement it equitably.
Christopher King
January 1, 2026 AT 20:17Think about it-what if your genes were programmed by someone else? Who decided which variants matter? Who funded the research? Who controls the database? The same corporations that make the drugs. This isn’t medicine-it’s algorithmic control dressed up as science.
They test your DNA to make sure you take the right pill… but what if the pill is the problem to begin with? What if the real solution isn’t tweaking your genes but changing the system that forces people to take 12 drugs at once?
And don’t tell me about ‘personalized medicine’-this is just another way to make you feel like your body is broken and needs fixing. Meanwhile, the real fix-better nutrition, less stress, access to care-is ignored because it doesn’t generate a patent.
They’re not saving lives with this. They’re monetizing fear.
Justin James
January 2, 2026 AT 07:55Okay so let me break this down for you because nobody else is willing to say it out loud-this whole pharmacogenetic testing thing? It’s a Trojan horse. They’re not doing it to save lives. They’re doing it to create a permanent genetic registry that ties every prescription you ever take to your DNA. And once that’s in the system, guess what? Insurance companies will start denying coverage based on your ‘high-risk’ gene profile. Employers will screen applicants. Even your future partner’s dating app might ask if you’re a ‘CYP2D6 ultra-rapid metabolizer’ before matching you.
And don’t tell me about ‘anonymized data’-that’s a lie. Data is never anonymized. It’s just encrypted until someone with enough money and a court order cracks it. The NSA already has your cousin’s cousin’s cousin’s genetic data from a 23andMe sale they never told you about.
And the FDA adding 42 new gene-drug pairs? That’s not science. That’s legal CYA. They’re covering their butts because they know someone’s going to die from a bad reaction and they’ll get sued. So they slap on a warning label and call it progress.
Meanwhile, in India, where I grew up, people take 15 pills a day and never get tested. And they live longer than we do. Coincidence? Or maybe our system is the problem, not our genes?
They want you to believe you’re broken. You’re not. The system is.